(note: patient must consent, no LAR consent allowed)
Inclusion criteria:
- Symptomatic brain infarction visible on imaging that occurred within 30 days
- Infarct attributed to 70-99% stenosis (or flow gap on MRA) of a major intracranial artery (carotid artery, MCA (M1 or M2), vertebral artery (V4), basilar artery, ACA (A1), or PCA (P1) documented by CTA, MRA, or catheter angiography. (see separate instructions for measuring stenosis – CONFIRM MEASUREMENT WITH SCOTT OR BRETT PRIOR TO RANDOMIZATION)
- mRS ≤ 4
- Ability to swallow pills
- > 30 years old – if 30-49 years required to meet at least one of the following additional criteria:
- diabetes treated with insulin for at least 15 years
- at least 2 of the following atherosclerotic risk factors: hypertension (BP > 140/90 or on antihypertensive therapy); dyslipidemia (LDL > 130 mg /dl or HDL < 40 mg/dl or fasting triglycerides > 150 mg/dl or on lipid lowering therapy); smoking; non-insulin dependent diabetes or insulin dependent diabetes of less than 15 years duration; any of the following vascular events occurring in a parent or sibling who was < 55 years of age for men or < 65 for women at the time of the event: myocardial infarction, coronary artery bypass, coronary angioplasty or stenting, stroke, carotid endarterectomy or stenting, peripheral vascular surgery for atherosclerotic disease
- personal history of any of the following: myocardial infarction, coronary artery bypass, coronary angioplasty or stenting, carotid endarterectomy or stenting, or peripheral vascular surgery for atherosclerotic disease
- any stenosis of an extracranial carotid or vertebral artery, another intracranial artery, subclavian artery, coronary artery, iliac or femoral artery, other lower or upper extremity artery, mesenteric artery, or renal artery that was documented by non-invasive vascular imaging or catheter angiography and is considered atherosclerotic
- aortic arch atheroma documented by non-invasive vascular imaging or catheter angiography
- any aortic aneurysm documented by non-invasive vascular imaging or catheter angiography that is considered atherosclerotic
- Negative pregnancy test if able to become pregnant
- Subject has provided informed consent (use of a LAR is not permitted)
- aortic arch atheroma documented by non-invasive vascular imaging or catheter angiography or atherosclerotic aortic aneurysm
Exclusion criteria:
- Previous treatment of target lesion with a stent, angioplasty, or other mechanical device, including mechanical thrombectomy for the qualifying stroke, or plan to perform one of these procedures
- Plan to perform concomitant angioplasty or stenting of an extracranial vessel tandem to the symptomatic intracranial stenosis
- Intracranial tumor (except meningioma) or any intracranial vascular malformation
- Thrombolytic therapy within 24 hours prior to randomization
- Progressive neurological signs within 24 hours prior to randomization
- History of any intracranial hemorrhage (parenchymal, subarachnoid, subdural, epidural)
- asymptomatic radiographic microhemorrhages or hemorrhagic conversion of infarction are not exclusions but the latter requires delaying randomization for 2 weeks from onset of qualifying stroke
- Intracranial arterial stenosis due to arterial dissection; MoyaMoya disease; any known vasculitic disease; herpes zoster, varicella zoster or other viral vasculopathy; neurosyphilis; any other intracranial infection; any intracranial stenosis associated with CSF pleocytosis; radiation induced vasculopathy; fibromuscular dysplasia; sickle cell disease; neurofibromatosis; benign angiopathy of central nervous system; postpartum angiopathy; suspected vasospastic process; reversible cerebral vasoconstriction syndrome (RCVS); suspected recanalized embolus
- Any cardiac source of embolism: Afib, mitral stenosis, mechanical valve, endocarditis, intracardiac clot or vegetation, MI < 3 months, left atrial spontaneous echo contrast
- Known allergy or contraindication to aspirin, rivaroxaban, clopidogrel, or ticagrelor
- Active peptic ulcer disease, major systemic hemorrhage within 30 days prior to randomization, active bleed or bleeding diathesis, platelets < 100,000, hematocrit < 30, INR > 1.5, clotting factor abnormality that increases the risk of bleeding, current alcohol or substance abuse, uncontrolled severe hypertension (systolic pressure > 180 mm Hg or diastolic pressure > 115 mm Hg), severe liver impairment (AST or ALT > 3 x normal, cirrhosis), or CrCl < 15 mL/min or on dialysis
- Major surgery (including open femoral, aortic, or carotid surgery, cardiac) within 30 days prior to randomization or planned within 90 days after randomization
- Any condition other than intracranial arterial stenosis that requires the subject to take any antithrombotic medication other than aspirin (NOTE: exceptions allowed for subcutaneous heparin for deep vein thrombosis (DVT) prophylaxis while hospitalized)
- Severe neurological deficit that renders the subject incapable of living independently
- Dementia or psychiatric problem that prevents the subject from following an outpatient program reliably
- Co-morbid conditions that may limit survival to less than 12 months
- Pregnancy or of childbearing potential and unwilling to use contraception for the duration of this study, or currently breastfeeding. If a subject becomes pregnant during the course of the study, investigational product should be discontinued immediately
- Current or anticipated concomitant oral or intravenous therapy with strong CYP3A4 inhibitors or CYP3A4 substrates that cannot be stopped (list of drugs in Appendix)
Rules for Measurement of Percent Stenosis of Intracranial Arteries
- Vessel size must be done using an electronic cursor provided by the imaging software
- The image showing the most severe diameter stenosis should be chosen for measuring the stenotic diameter (Ds)
- To measure the diameter of the reference normal vessel (Dn) use the following WASID rules for establishing where to measure Dn.
Location for measuring Dn for the MCA
1st choice: the non-diseased, proximal part of the ipsilateral MCA at its widest, non-tortuous segment that has parallel margins.
2nd choice: the non-diseased, distal part of the ipsilateral MCA at its widest, parallel, non-tortuous segment. Use 2nd choice if the proximal artery cannot be used e.g., MCA origin stenosis.
3rd choice: the non-diseased, most distal, parallel, non-tortuous segment of the ipsilateral supraclinoid carotid artery. Use the 3rd choice if the entire MCA is diseased
Location for measuring Dn for the Intracranial Carotid Artery
1st choice: the non-diseased, widest, parallel, non-tortuous portion of the petrous carotid artery
2nd choice: the most distal, parallel part of the extracranial internal carotid artery. Use 2nd choice if the entire petrous carotid is diseased
Location for measuring Dn for the Intracranial Vertebral Artery
1st choice: the non-diseased, proximal part of the ipsilateral intracranial vertebral artery at its widest, non-tortuous segment that has parallel margins.
2nd choice: the non-diseased, distal part of the ipsilateral vertebral artery at its widest, parallel, non-tortuous segment. Use 2nd choice if the proximal artery cannot be used e.g., stenosis at the most proximal part of the vertebral artery
3rd choice: the non-diseased, most distal, parallel, non-tortuous segment of the ipsilateral extracranial vertebral artery. Use the 3rd choice if the entire intracranial vertebral artery is diseased
Location for measuring Dn for the Basilar Artery
1st choice: the non-diseased, proximal part of the basilar artery at its widest, non-tortuous segment that has parallel margins.
2nd choice: the non-diseased, distal part of the basilar artery at its widest, parallel, non-tortuous segment. Use 2nd choice if the proximal basilar cannot be used e.g., stenosis at the most proximal part of the basilar artery
3rd choice: the non-diseased, most distal, parallel, non-tortuous segment of the dominant vertebral artery. Use the 3rd choice if the entire basilar artery is diseased
See figure on the other side for where to measure Dn for examples of carotid and basilar stenoses.
By using the formula (1 – [D s / Dn]) x 100), the percent diameter stenosis of the target lesion will be calculated.
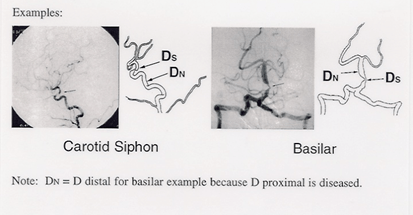
For carotid siphon lesion, D normal = diameter of petrous carotid artery at widest, parallel, non-tortuous
